LEONI JOUBERT (M.Phil (Optom) (RAU); B.Optom (RAU); MBCO(UK); CAS (NECO-USA); CAS (BV and Paeds NECO-USA); SAALED; Cert Dyslexia (UJ))
In a large meta-analysis undertaken by Holden et al (2016) and covering data from 145 studies which involved 2.1 million participants they estimated that 1,4 billion people worldwide had myopia in 2000. This was approximately 23% of the world’s population. Of these, 163 million people had high myopia (classified as having -5.00DS of myopia or worse) and this was 2.7% of the world population.
They predict that by 2050 there will be 4,758 billion people with myopia which will be about 50% of the world’s population and there will be 938 million people with high myopia (9.8% of the world’s population). Marcus et al (2011) stated that there were 1,6 billion people with myopia in 2011 and that this would increase to 2,5 billion by 2020. In East Asia the prevalence of myopia is already as high as between 80 and 96% of the population! (Jong, 2017).
Although there is no universal consistency on the classification of myopia, for the purposes of this article myopia will be classified as follows:
Very Low Myopia (<1.00D), Low Myopia (-1.00D to -3.00D), Moderate Myopia (-3.00D to -5.00D), High Myopia (-5.00D to -9.00D) and Very High Myopia (> -9.00D) (Michaud, 2016). Pathological Myopia is defined as myopia > -6.00D or and axial length of > 26.5mm (Friedman, 2007).
In the United States alone the annual direct cost of compensating for distance vision impairment due to refractive errors is between US$3.9 and US$7.2 billion per annum according to the Health and Nutrition Examination Survey (NHANES) (Pan, 2012).
Besides the cost involved in compensating for this myopia a far greater threat looms where myopia is concerned. There are a number of serious ocular complications related to myopia. These include open angle glaucoma (OAG), chorioretinal changes including retinal detachment, sub-retinal neovascularization, maculopathy and cataract formation.
This article will briefly outline some of these conditions. Practitioners should be aware that it is not only patients with high myopia that might present with these conditions but even people with so-called mild myopia that could develop them.
- Open Angle Glaucoma:
Open-angle glaucoma is a defined as the progressive atrophy of the optic nerve in the presence of an open angle. It is accompanied by changes in the appearance of the optic disc, changes to the nerve fibre layer, loss of peripheral vision and is usually associated with elevated intra-ocular pressure. Open-angle glaucoma is the most common form of glaucoma and is responsible for approximately 12% of global blindness.
Individuals with myopia have an approximately doubled risk of developing OAG in comparison with individuals without myopia (Marcus, 2011). Some authors show an even higher risk of 3-4 times for individuals with myopia for developing OAG (Gifford, 2013; Mitchell, 1999; Detry-Morel, 2010).
When one thinks of a myopic eye which is usually myopic by virtue of the fact that it is elongated and the structures such as the anterior and vitreous chambers are larger, one would not expect glaucoma to occur as the anterior chamber depth is usually also larger. However, in myopic eyes the lamina cribrosa is deformed and this might be the mechanism for the development of OAG as it makes the eye more susceptible to glaucomatous optic nerve changes (Marcus, 2011).
Another promising theory is that shown by Chen et al (2018) which showed that there are structural changes in both Schlemm’s canal as well as the trabecular meshwork in high myopes. They defined any myopia >-6.00D or axial length>26.5mm as high or pathological myopia. This might affect the outflow of aqueous humor and therefore lead to an increase in intra ocular Pressure (IOP) which is the driving force behind OAG.
Other structural changes that occur in the optic nerve of myopes include a higher cup–disc ratio (CDRs) and increased optic nerve fibre layer defects (Saw, 2005). Myopic patients have also been shown to have significantly higher rates of larger, tilted, rotated discs, larger disc areas, longer disc to foveola distances and larger long: short axis ratios. These anatomical differences in the optic nerves and related areas are important clinically because they make the optic nerve head area difficult to evaluate. The detection of both glaucoma and progression of glaucomatous optic neuropathy may therefore be delayed in myopic patients.
There seems to be a correlation between the degree of myopia and the risk of developing glaucoma, the higher the myopia the greater the risk of OAG (Saw, 2005).
In all instances of myopia is it imperative to carefully monitor the patient for any signs of OAG and to bear in mind the structural differences when evaluating the optic nerve area.
- Chorioretinal abnormalities including Retinal Detachment:
The elongation that occurs in myopia leads to thinning and stretching of the choroid and retinal pigment epithelium, this can lead to vascular and degenerative changes in the retina and choroid. Several studies have shown that this thinning found in myopia can lead to retinal breaks, retinal detachment, chorioretinal atrophy, Fuch’s spot, lacquer cracks, pigmentary degeneration, lattice degeneration, posterior staphyloma and white without pressure (Saw 2005). There is also a risk of choroidal neovascularization and macular holes (Lai, 2016).
A study done by The Eye Disease Case-Control Study Group (1993) states that “An eye with a spherical equivalent refractive error of −1 to −3 diopters had a fourfold increased risk of retinal detachment compared with a non-myopic eye and that if the refractive error was greater than −3 diopters, the risk was increased 10-fold.” They also mention that almost 55% of non-traumatic detachments in eyes without previous surgery are attributable to myopia and that the etiology of retinal detachment appears to be related to the architecture of the eye.
Staphyloma and chorio-retinal atrophy are the most frequent myopic retinopathy signs (observed in about 80% and 56% of individuals with pathologic myopia, respectively (Verkicharla, 2015).
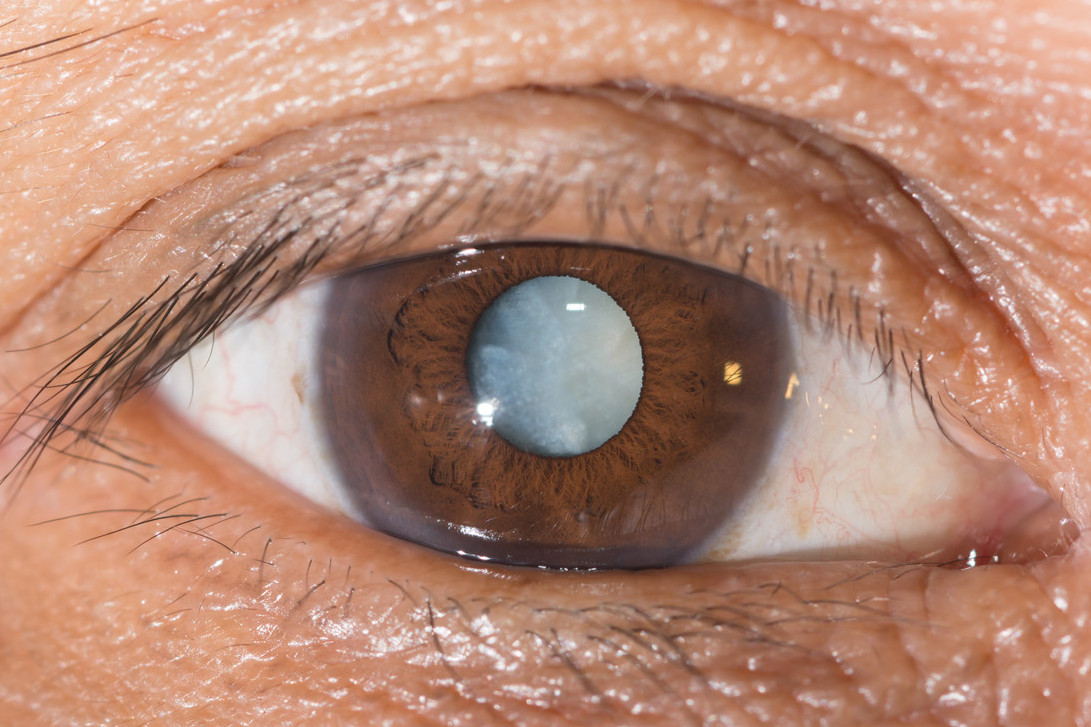
- Cataract:
It is well-known that cataract is the leading cause of blindness worldwide (Resnikoff et al., 2004). Some studies show a risk of cataract development due to myopia and the risk seems to be related to the degree of myopia. However other studies found no associated risk. One must bear in mind though that the development of a cataract (specifically a nuclear cataract) can lead to myopic shift and this then means that the cataract–myopia relationship may be in the opposite direction and the progression of opacity in the lens nucleus may initiate the development of myopia and not the other way around (Lim et al., 1999). One cannot then infer that cataract is a complication of myopia because it is also possible that adults with cataract may have a higher risk of myopia.
It appears that the mechanism by which myopia may lead to lens changes is unknown. A possible explanation might be that myopia may lead to damage of rod outer segments and increased production of potentially cataractogenic lipid peroxidation by-products (Zigler et al., 1983).

- Myopic Macular Degeneration (MMD):
Once again what defines “Myopic Macular Degeneration” has not been consistent across studies hence classification is difficult. For the purpose of this article MMD will be meant to include the presence of at least one of the following: diffuse chorioretinal atrophy at the posterior pole, patchy chorioretinal atrophy, lacquer cracks, or macular atrophy, staphyloma, Fuchs’ spot and myopic chorioretinal thinning (Ohno-Matsui, 2016).
The prevalence of MMD increases in a nonlinear pattern with increasing age, spherical equivalent refractive error and axial length. The pathogenesis of MMD has been postulated as being due to a decrease in choroidal circulation in high myopia. Narrowing and loss of large choroidal vessels have been observed in eyes with high myopia and loss of choroidal large vessels and fibrotic changes within choroidal vessels may lead to obstruction of choroidal blood flow and eventually be replaced with fibrous tissue. This would ultimately resulting in myopic chorioretinal atrophy (Ohno-Matsui, 2016).
MMD can result in irreversible loss of central vision and is one of the leading causes of blindness in countries worldwide (Wong, et al, 2018).
Conclusion:
Myopia can no longer be seen as an innocuous refractive error. There are serious long-term complications that can occur and that may impact the sight of an individual with myopia. As myopes age these complications become more prevalent. The prevalence of myopia is increasing dramatically world-wide and hence the long-term implication of myopic complications will only be exacerbated by the aging myopic population.
Pathologic myopia is the primary cause of low vision or blindness in approximately 7% of individuals in the population of Europe and 12–27% of individuals in the population of East Asia. Ageing is considered one of the important risk factors for pathologic myopia (Verkicharla, 2015). Ageing in combination with changes in environmental factors such as increasing urbanization are known to have a direct impact on the prevalence of myopia and pathologic myopia.
Optometric practitioners need to be vigilant in identifying myopia early on in children and attempting a myopic prevention strategy, or a method to reduce the progression of myopia, in order to reduce the risk of serious visual complications later on in life. In current myopic patients practitioners need to carefully monitor them for any pathologic changes and set up intervention strategies to preserve sight as best as possible.
References:
Chen Z, Song Y, Li M, Chen W, Chen L and Xiang Y. Schlemm’s Canal and trabecular meshwork morphology in high myopia. Ophthalmic and Physiological Optics Volume 38, Number 3. May 2018
Detry-Morel M1. Is myopia a risk factor for glaucoma? J Fr Ophtalmol. 2011 Jun;34(6):392-5. doi: 10.1016/j.jfo.2011.03.009. Epub. 2011.
The Eye Disease Case-Control Study Group, Risk Factors for Idiopathic Rhegmatogenous Retinal Detachment, American Journal of Epidemiology, Volume 137, Issue 7 pp749–757. April 1993
Friedman, NJ and Kaiser, PK. Retina. Essentials of Ophthalmology. Philidelphia: Elsievier Health Sciences; 2007. Pp 253-254.
Gifford K. Practitioners Guide to Clinical Myopia Profile. https://myopiaprofile.com/. 2013.
Jong, M; Sankaridurg, P; Tan, K. The Business of Managing Myopia. http://www.mivision.com.au/, 2017).
Lai T, Cheung CM/ Myopic Choroidal Neovascularization: Diagnosis and treatment. Retina (36(9)):1614-1621. September 2016.
Lim, R., Mitchell, P. and Cumming, R. G. Refractive associations with cataract: the blue mountains eye study. Invest. Ophthalmol. Vis. Sci. 40, 3021–3026. 1999.
Marcus, M.W; de Vries, M; Montolio, F.G; Jansonius, N.M. Myopia as a Risk Factor for Open-Angle Glaucoma: A Systematic Review and Meta-Analysis. Ophthalmology Volume 118, Number 10. October 2011.
Michaud, L; Simard, P; Marcotte-Collard, R. Definig a strategy for myopia control. Contact Lens Spectrum. March 2016.
Mitchell P1, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. Oct;106(10):2010-5. 1999.
Ohno-Matsu,i K; Lai, TYY; Lai, CC. Updates of pathologic myopia. Progress in Retinal and Eye
Research 52. 2016.
Resnikoff, S., Pascolini, D., Etya’ale, D., Kocur, I., Pararajsegaram, R., Pokarel, G. and Mariotti, S. (2004) Global data on visual impairment in the year 2002. Bull World Health Organ 82, 844–51.
Saw SM, Gazzard G, Shih-Yen EC and Chua WH. Myopia and associated pathological complications. Ophthal. Physiol. Opt. 2005 25: 381–391
Shiel, JC. Medical Definition of Open Angle Glaucoma. Medicinenet. https://www.medicinenet.com/script/main/art.asp?articlekey=22379, 2018.
Verkicharla PW, Ohno-Matsui K and Saw SM. Current and predicted demographics of high myopia and an update of its associated pathological changes. Ophthalmic & Physiological Optics 35 pp 465–475. 2015.
Wong, YL; Sabanayagam, C; Ding, Y; Wong, C; Yeo, AC; Cheung,Y ; Cheung G; Chia , A; Ohno-Matsui, K; Wong, T; Wang, J; Cheng, C; Hoang, QV; Lamoureux, and Saw, S. Prevalence, Risk Factors, and Impact of Myopic Macular Degeneration on Visual Impairment and Functioning Among Adults in Singapore. IOVS Vol. 59 j No. 11. September 2018.
Zigler JS, Bodaness RS , Gery I. and Kinoshita JH. Effects of lipid peroxidation products on the rat lens in organ culture: a possible mechanism of cataract initiation in retinal degenerative disease. Arch. Biochem. Biophys. 225,149–156. 1993.